Abstract
Introduction: Acute Myeloid Leukemia (AML) is an aggressive neoplastic disorder with poor outcomes in children and adults. NK cell adoptive transfer is an anti-cancer immunotherapy that has promise for AML treatment. We aimed to improve NK cell anti-tumor efficacy with expression of a Chimeric Antigen Receptor (CAR) on the cell surface. Our CAR consists of an extracellular single-chain variable fragment targeting the AML-associated antigen CD123 (IL3Rα) and intracellular domains derived from 2B4 and TCRζ. We sought to improve the persistence and long-term functionality of our CAR-NKs by introducing transgenic interleukin-15 (IL15).
Methods: CD3-depleted PBMCs were first activated with lethally irradiated feeder cells, then transduced with transiently produced replication incompetent γ-retrovirus (αCD123.2B4.ζ, αCD123.2B4.ζ-IRES-sIL15, sIL15-IRES-mOrange) on day 4 of culture. CAR expression was measured on day 8 using FACS. Secretion of IL15 was verified with ELISA. Cytotoxicity was measured using ffLuc expressing target cells and bioluminescence (BL) measurement. In serial stimulation assays, target cells were repleted daily to maintain a 1:1 effector:target ratio. Immunophenotype and cell counts were assessed by FACS. Transcriptomic analysis (RNAseq) was performed on RNA derived from NK cells purified on D10. Xenograft modeling was performed using NSG mice engrafted with MV-4-11.ffLuc or MOLM-13.ffLuc AML cell lines. Mice were treated with NK cells on D4 or D4-7-10. Untreated mice served as controls. Tumor growth was serially tracked in vivo using BL imaging. NK cell persistence and expansion were measured in peripheral blood.
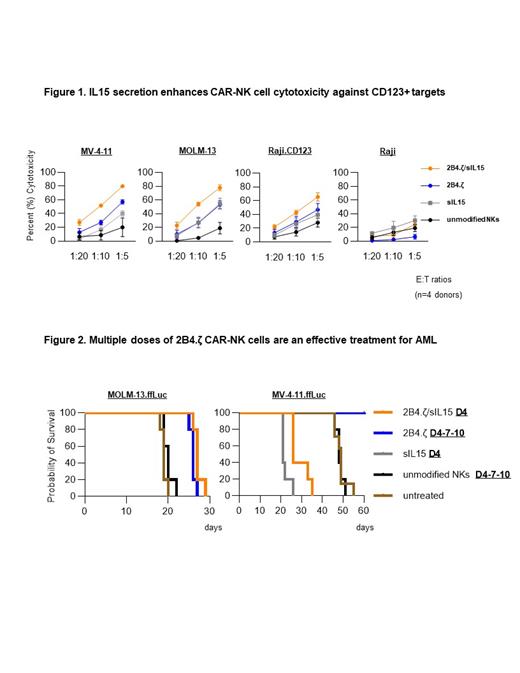
Results: The 2B4.ζ CAR was well expressed on the surface of transduced NK cells (median transduction efficiency 95%, range 85-97%, n=3). 2B4.ζ CAR-NK treatment prolonged survival of AML engrafted mice when compared to treatment with unmodified NKs (median survival: 63 vs 55 days; n=8 mice; p=0.014). Serial peripheral blood analysis revealed a steady decline in circulating NK cells, which were undetectable in all cohorts within 21 days. NK cells were then engineered for constitutive secretion of IL15, with and without CAR expression. 2B4.ζ/sIL15 CAR-NKs had the most potent 24h-cytotoxicity against CD123+ targets (Fig. 1). After a 10-day chronic stimulation with MV-4-11, 2B4.ζ/sIL15- and sIL15-NKs expanded (x1.2 and x5.9 respectively), while NK cells without sIL15 decreased in number. In this assay, only 2B4.ζ/sIL15 CAR-NKs exhibited sustained tumor killing. Transcriptomic analysis after 10 days of serial stimulation showed sample clustering dependent on IL15 secretion. Differential gene expression analysis (DESeq2) identified upregulation of genes associated with cell cycle progression, apoptosis regulation, chemokine signaling, and NK cell mediated cytotoxicity in NK cells secreting IL15 compared to those without. In multiparameter flow cytometric analysis, 2B4.ζ/sIL15 CAR-NKs had a higher percentage of NK cells populating clusters defined by higher surface expression of NK cell activating receptors (NKp30, NKG2D, LFA-1) compared to 2B4.ζ and unmodified NK cells. In our MV-4-11 xenograft model, NKs armed with secreted IL15 expanded in vivo and had improved persistence. A single dose (D4) of 2B4.ζ/sIL15 CAR-NKs demonstrated an initial antitumor response, equivalent to that seen following 3 doses (D4-7-10) of 2B4.ζ CAR-NKs. However, mice treated with IL15-secreting NKs had short survival (Fig. 2). Compared to control mice, peripheral blood analysis showed increasing systemic hIL15 and higher levels of hTNFα. In our more aggressive MOLM-13 xenograft model, both single dose 2B4.ζ/sIL15 CAR-NK and multiple dose 2B4.ζ CAR-NK treatment prolonged survival compared to treatment with unmodified NKs. (27 and 26 vs 20 days; n=5 mice; p<0.01; Fig. 2).
Conclusion: 2B4.ζ CAR-NKs have limited antitumor efficacy and short persistence in vivo. NK cells armored with secreted IL15 have enhanced anti-AML cytotoxicity and in vitro persistence. Introduction of IL15 secretion confers a distinctly activated phenotype that is maintained during chronic antigen stimulation. Constitutive local IL15 secretion improves in vivo NK cell persistence but may cause lethal toxicity when employed against AML. These results warrant further study and should impact the development of CAR-NK clinical products for patients with AML.
Ho: Rodeo Therapeutics/Amgen: Patents & Royalties; Exelixis: Consultancy; Sanofi: Research Funding. Bonifant: Kiadis Pharma: Research Funding; BMS: Research Funding; Merck, Sharpe, Dohme: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal